Congratulations to Leanna, David, and Yidi on their collaboration with Margot Riggi and Janet Iwasa to create a molecular animation depicting the full progression of clathrin-mediated endocytosis in budding yeast, along with an accompanying Cell Science at a Glance article and poster. Read the review here.
Category Archives: Uncategorized
Au revoir, Paul!
Goodbye Dehbia! We will miss you!
Congratulations to our graduated undergraduates!
Paper by Ross Pedersen on functional role of endocytic myosins now published in JCB
Congratulations to Ross Pedersen on his new paper “Type I myosins anchor actin assembly to the plasma membrane during clathrin-mediated endocytosis“, now published in the Journal of Cell Biology.
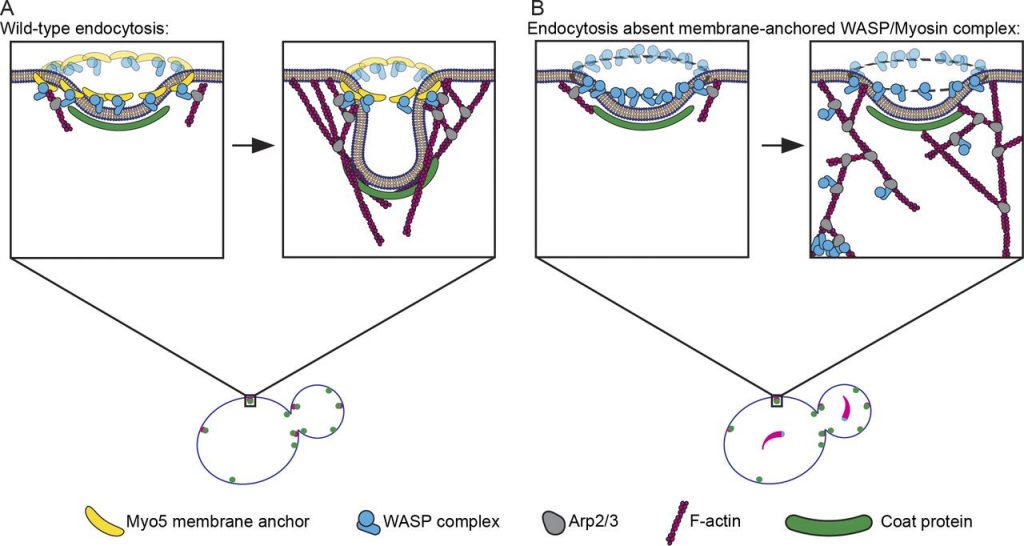
Model for Myo5 function in anchoring actin assembly to the PM at endocytic sites. (A) Myo5 (yellow bananas) restricts activation of the Arp2/3 complex (gray avocados) by the WASP complex (blue widgets) to a discrete location, generating an actin array that grows predominantly in the same direction to generate force. (B) Absent this critical linkage, Arp2/3 activators splinter off of the PM, leading to Arp2/3 complex activation throughout the actin network. Delocalized Arp2/3 complex activation results in disordered actin arrays that fail to produce force. In the most catastrophic cases, the Arp2/3 complex and its activators pull away from the PM completely to form cytoplasmic actin comets (lower left of zoom).
Paper by Joh on analysis of lattice light sheet imaging data now published online in MBoC
Congratulations to Joh and former lab member Daphne on their paper “4D cell biology: big data image analytics and lattice light-sheet imaging reveal dynamics of clathrin-mediated endocytosis in stem cell derived intestinal organoids“, now published online in Molecular biology of the cell.
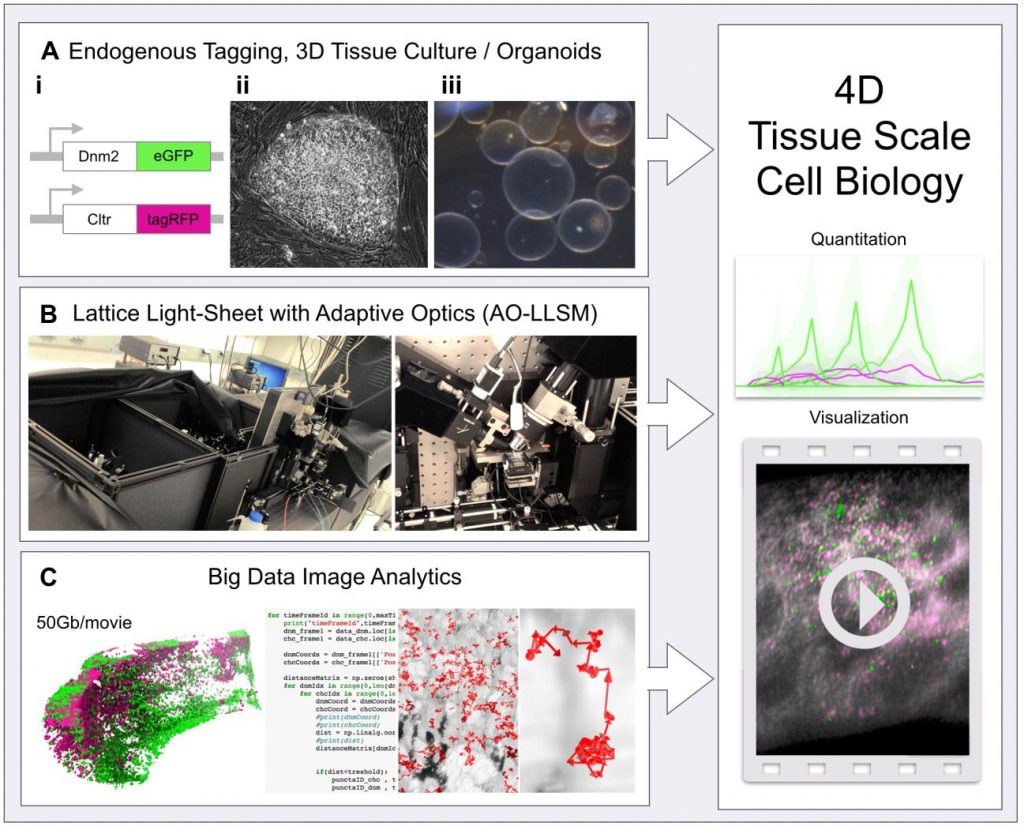
A new level of 4D tissue cell biology is unlocked as recent advances from three fields come together. (A) Endogenous protein tagging using genome editing (i), stem cell biology (ii) and 3D tissue/organoid culture (iii), (B) 4D non-invasive advanced fluorescent imaging with the lattice light-sheet microscope with adaptive optics (AO-LLSM, left: full view of the microscope, right: focus on the characteristic objective arrangement) and (C) advances and software in big data image analytics. (Right) Combination of these elements allows unprecedented quantitative analysis of subcellular events within live tissues in 4D.
Paper by Zane and Jonathan on microtubule dynamics accepted by JCS
Congratulations to Zane and Jonathan on their paper “Microtubule dynamics regulation reconstituted in budding yeast lysates“, now accepted and online in the Journal of Cell Science.
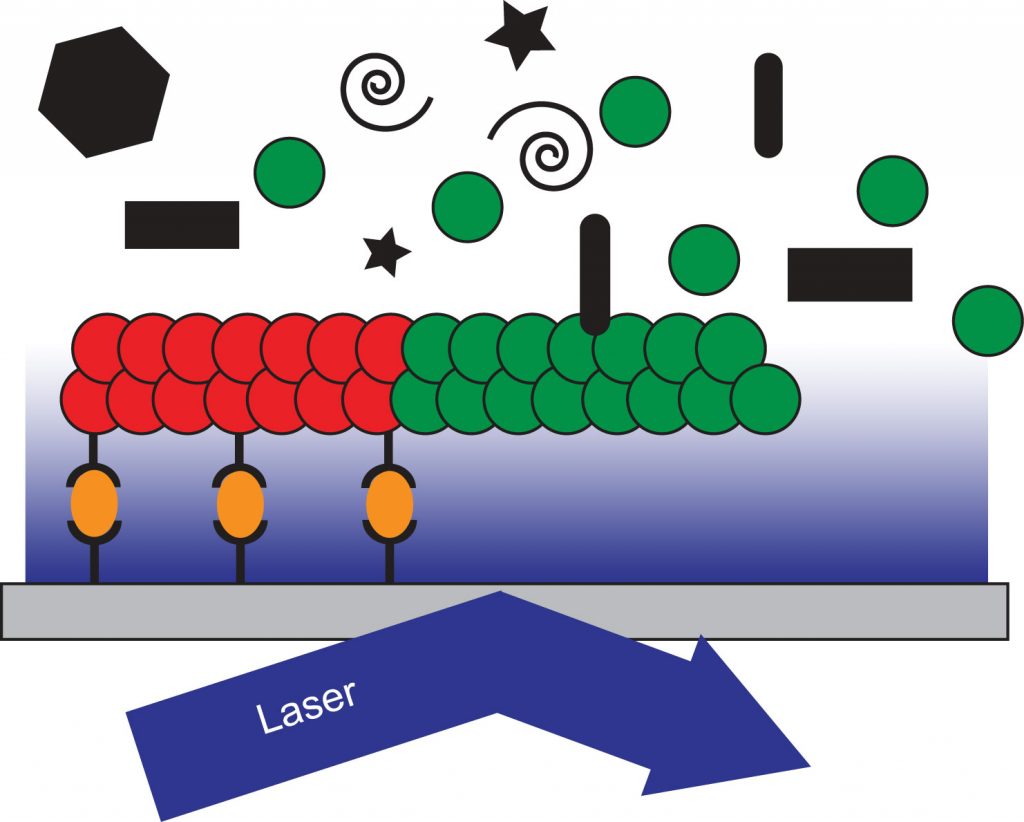
Recreation of Microtubule Dynamics in Budding Yeast Lysate. A reaction chamber is prepared by adhering GMPCPP-stabilized, rhodamine-labeled porcine microtubule seeds (red) to a glass coverslip through a biotin (black stems)-neutravidin (orange ovals) system. Whole-cell lysate from GFP-tubulin (green) expressing yeast strains is flowed into the chamber and incubated to allow for the polymerization of microtubules. Growth and dynamics of single microtubules is observed by TIRF microscopy in the context of other soluble proteins found in the cell (black shapes).
Paper by Daphe on differences in CME in stem cells and differentiated cells now published
Congratulations to Daphne on her paper “Genome-edited human stem cells expressing fluorescently labeled endocytic markers allow quantitative analysis of clathrin-mediated endocytosis during differentiation“, now published in the Journal of Cell Biology.
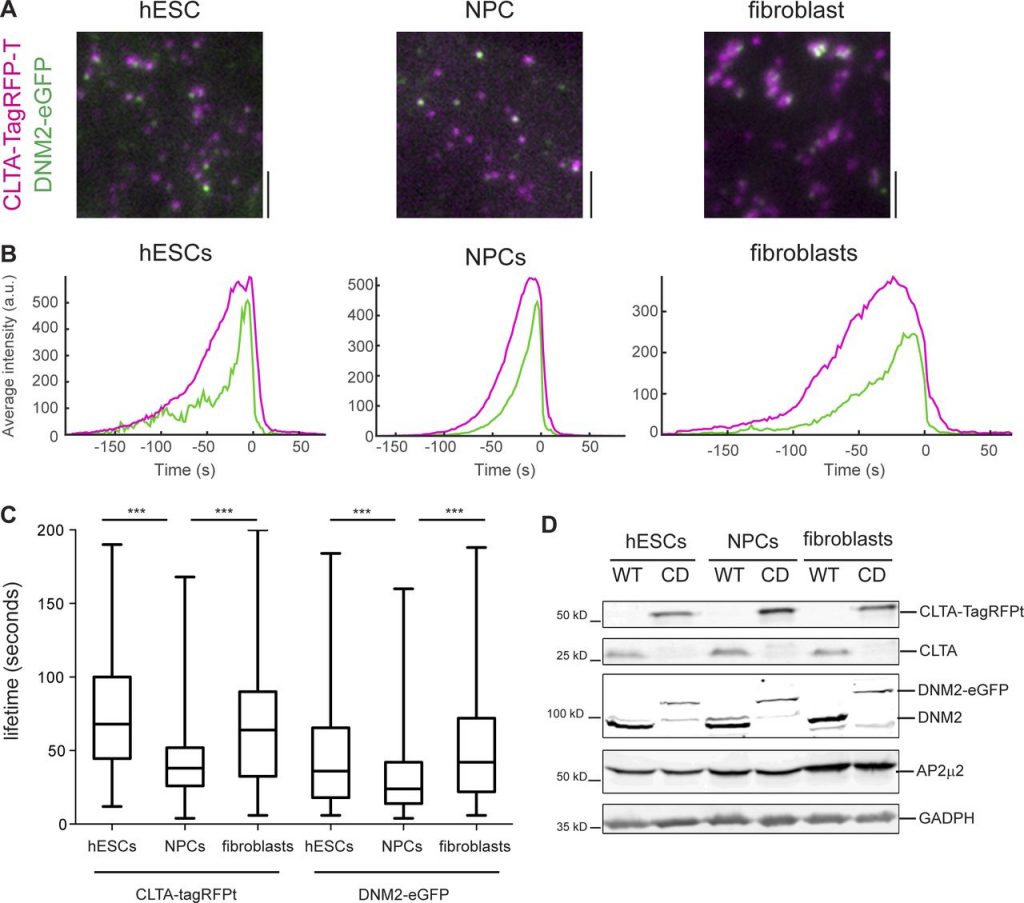
Endocytic dynamics in three isogenic cell types.
Paper by Itziar now published
Congratulations to Itziar on her paper “Kinesins relocalize the chromosomal passenger complex to the midzone for spindle disassembly” which is now published in the Journal of Cell Biology.
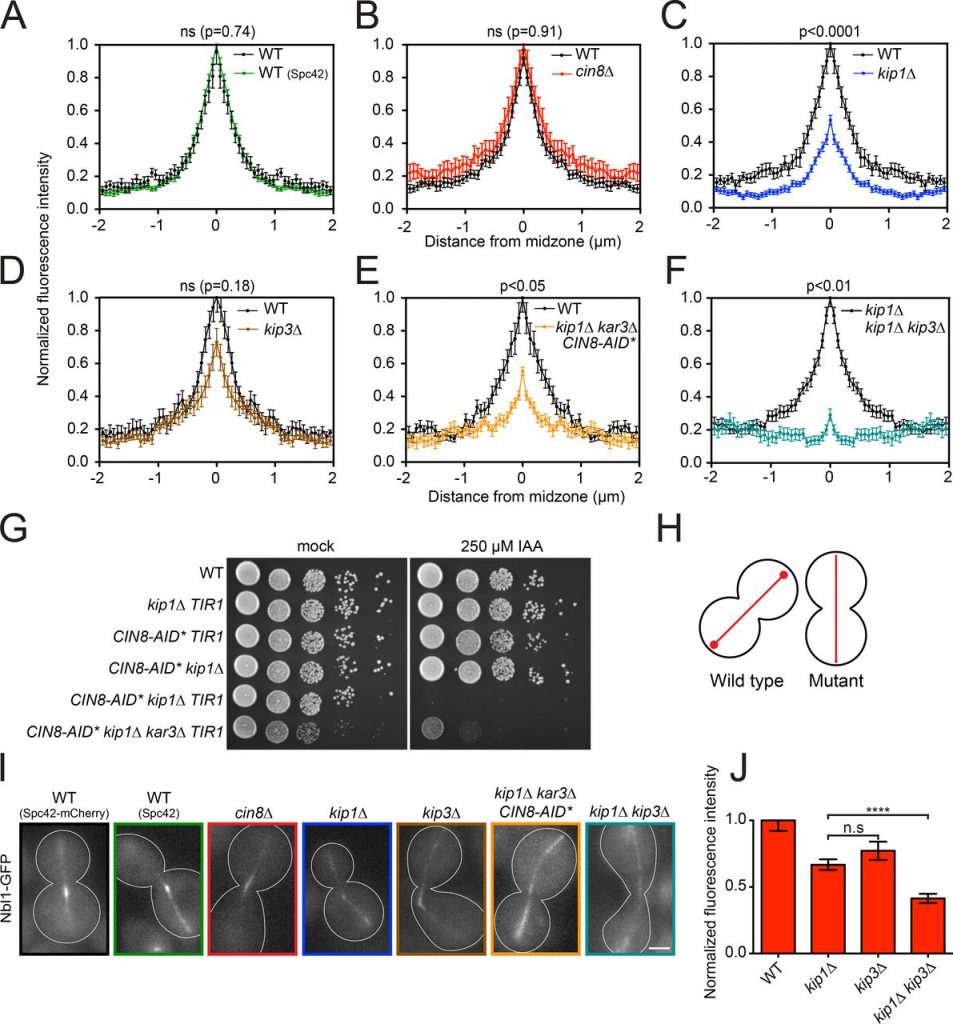
The CPC cannot concentrate at the spindle midzone in the absence of Kip1 and Kip3.
Goodbye Jessica!
The Drubin/Barnes lab celebrated post-doc Jessica Marks moving on to her new position as a AAAS Fellow. We wish her all the best in Washington, DC!

Celebration at Brazil Cafe as Jessica moves to a new position a AAAS Fellow in D.C.




